Shipping Training is Required Every Two Years to Package and Ship Hazardous Materials.
- Hazardous Materials shipping training modules for (1) General Awareness and (2) Dry Ice are available at the UC Learning Center. Search for "hazmat," click the needed course and use the "Start" button to launch the training.
- The Centers for Disease Control and Prevention provides a free online and on-demand training course on packing and shipping Division 6.2 infectious substances and dry ice, and a certificate of completion.
- Packing and Shipping Dangerous Goods: What the Laboratory Staff Must Know
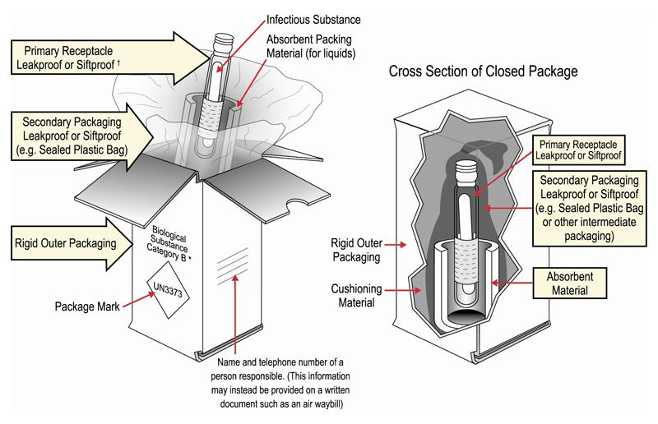
Triple Packaging is Required for All Regulated Biological Shipments.
Three separate layers of packaging present multiple barriers that decrease the likelihood of exposure if an incident occurs with a package containing biological substances.
- A leakproof primary receptacle (such as a sealed cryovial, test tube, etc.)
- A leakproof secondary packaging
- Outer packaging of adequate strength for its capacity, weight, and intended use
For shipments of liquids, there must be sufficient absorbent placed inside the secondary packaging to absorb the entire contents of the primary receptacle(s) should leakage occur. Packaging sets may be reused however you as the shipper are required to retain a copy of the manufacturer’s instructions for a minimum of 90 days from the time the shipment is offered for transport.
Contact the UCSB Export Control Officer prior to all international shipments, regardless of the shipment category, for review of the technology and intended recipients: Exportcontrol@research.ucsb.edu and
(805) 893-3787
Biological Substances are Assigned to One of the Following Categories:
|
Unrestricted biological materials are those materials that are known to be free of pathogens, or are expressly excepted from the regulations. No UN number No proper shipping name No packing instructions Examples:
|
(Excepted from the regulations) |
|
Genetically modified organisms (GMO) and micro-organisms (GMMO) are capable of altering animals, plants or microbiological substances in a way which is not normally the result of natural reproduction. NOTE: Pathogenic GMO or GMMO must be appropriately classified as either Category A or Category B Infectious substances. |
|
|
Exempt Patient Specimens are substances collected directly from humans or animals and transported for research, diagnosis, investigational activities or disease treatment or prevention. To classify a substance as an Exempt Patient Specimen, professional judgment must be used to determine whether there is a minimal likelihood that the specimen contains a pathogen. The only mark required on the package is either "Exempt Human Specimen" or "Exempt Animal Specimen,”i.e., there is no diamond hazard label. In the visual shown at right, specimens are packed with dry ice, hence the package is marked and labeled for dry ice in addition to "Exempt human specimen." |
|
|
Category B, Infectious Substance - moderately hazardous and/or potentially pathogenic substances. UN3373 (on hazard label) Packing Instruction 650 Examples:
|
|
|
Category A Infectious Substance - high consequence, high containment, life-threatening pathogens. UN2814 or UN2900 Proper shipping name: Infectious substance affecting humans or Infectious substance affecting animals Packing Instruction 620 |
|
The Biosafety Officer May Be Reached at biosafety@ehs.ucsb.edu and (805) 893-8894